Describe a Network Solid and Give Two Examples
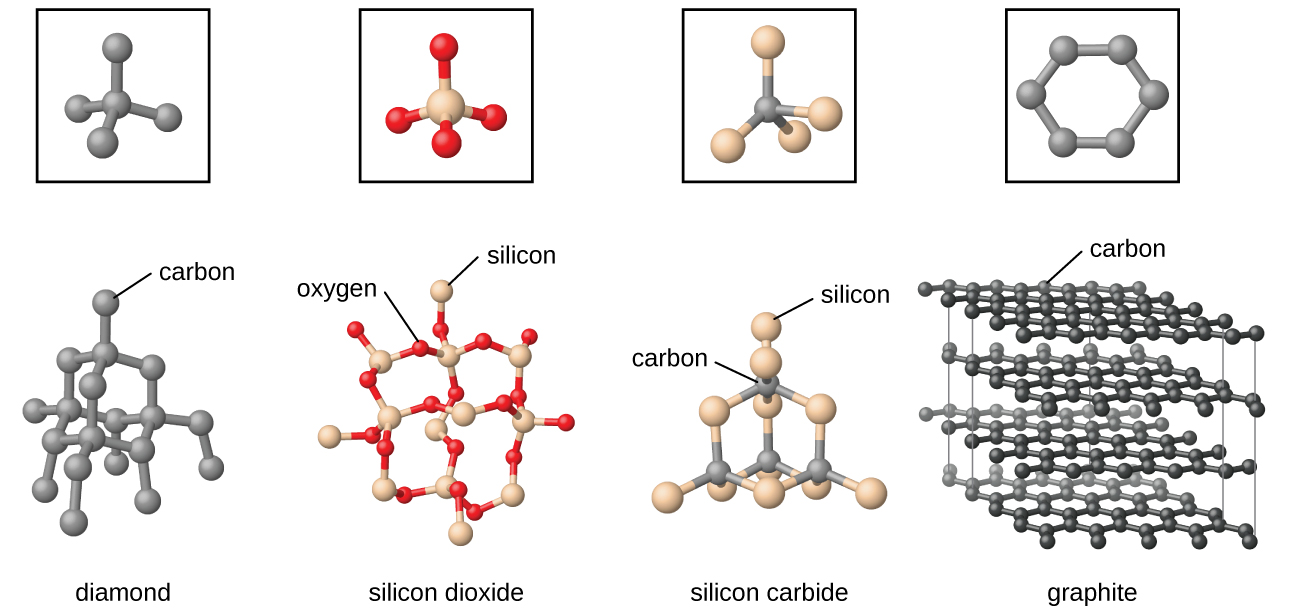
Examples of network-related covalent solids include diamond and also graphite both allotropes of carbon and also the chemical compounds silsymbol carbide and also boron-carbide. - 14537771 vdulce714 is waiting for your help.
Covalent compounds are the substance that is made generally by bonding between two or more non-metals.
. Characterized by low melting points and flexibility and are poor conductors. The surface areas of two similar solids are 340 yd2 and 1158 yd2 the volume of the larger solids is 1712 yd3 what is the volume of the smaller solid. Solutions for Chapter 14 Problem 47QP.
Describe a network solid and give two examples. Describe a network solid and give two examples. Network solids include diamond quartz many metalloids and oxides of transition metals and metalloids.
Give an example of a network solid and describe the bonding in such a solid. In diamond the bonding occurs in the tetrahedral geometry while in graphite the carbons bond with each other in the trigonal planar arrangement. And these atoms can be carbon for example which would make up graphite or diamond.
The smallest amount of a network solid that have the right to be determined as such is called a formula unit. Describe the role and. What is a global policy network.
In the below text I will. 163 Ionic Metallic and Network Condensed Phases CK-12. How does a network solid differ from a molecular solid.
Network solids are substances in which all of the atoms are covalently bonded to each other. Two Allotropes of CarbonThese two allotropes of carbon are covalent network solids which differ in the bonding geometry of the carbon atoms. Posted 7 months ago.
A silicon crystal is another example consisting of Si atoms. Identify the purpose and describe at least one activity performed in each of the following three phases in. Describe a network solid and give two examples.
NCERT Solutions for Class 12th Chemistry Chapter 1 The. Network Solid Examples. Give an example of a network solid and describe the bonding in such a solid.
Two examples would be diamond and graphite Molecules of carbon dioxide and water have different shapes even though they both have three atoms. There are many types of networks depending upon the geographical range. What are two examples of networks solids.
Diamonds are network solids made of carbon atoms. The two examples of network solid are diamond and silicon di oxideFurther explanationThe network solid is in amorphous shape with different molecules. A network solid is a chemical compound in which the atoms are covalently bonded to other atoms in continuous networks.
Computer networks are used to share files and data between computers like sharing file in office or sharing file from one city to other city. Solids in which all of the atoms are covalently bonded to each other as a result they are very stable. Network solids are hard and brittle with extremely high melting and boiling points.
Characterized as being very hard with very high melting points and being poor conductors. Melting these substances requires breaking covalent bonds throughout the solid. So these are examples of network solids where these are atoms and these are CO Vaillant Bonds Vaillant Bonds and these are Adams.
Make sure all the questions to the point not much length or extra description 1. If you want to share some files or data between two or more computers then we have make a network between computers. The covalently bonded network is three-dimensional and contains a very large number of atoms.
A 250 mL aliquot of a well-shaken and filtered sample of river is pipetted into an evaporating dish. What is a network solid. A network solid is a network compound in which atoms are compounded continuously by using the co-valent bond.
You meet and carried out a network solid and describe give two examples include polythene. So a network solid is this kind of structure that is held together with CO Vaillant bonds. They take higher temperatures to melt because melting them requires breaking these covalent bonds.
Two examples are diamond and silicon carbide. The intermolecular forces are covalent bonds as well. Graphite a consist of continuous two dimensional layers covalently bonded within the layer with other bond types holding the layers together.
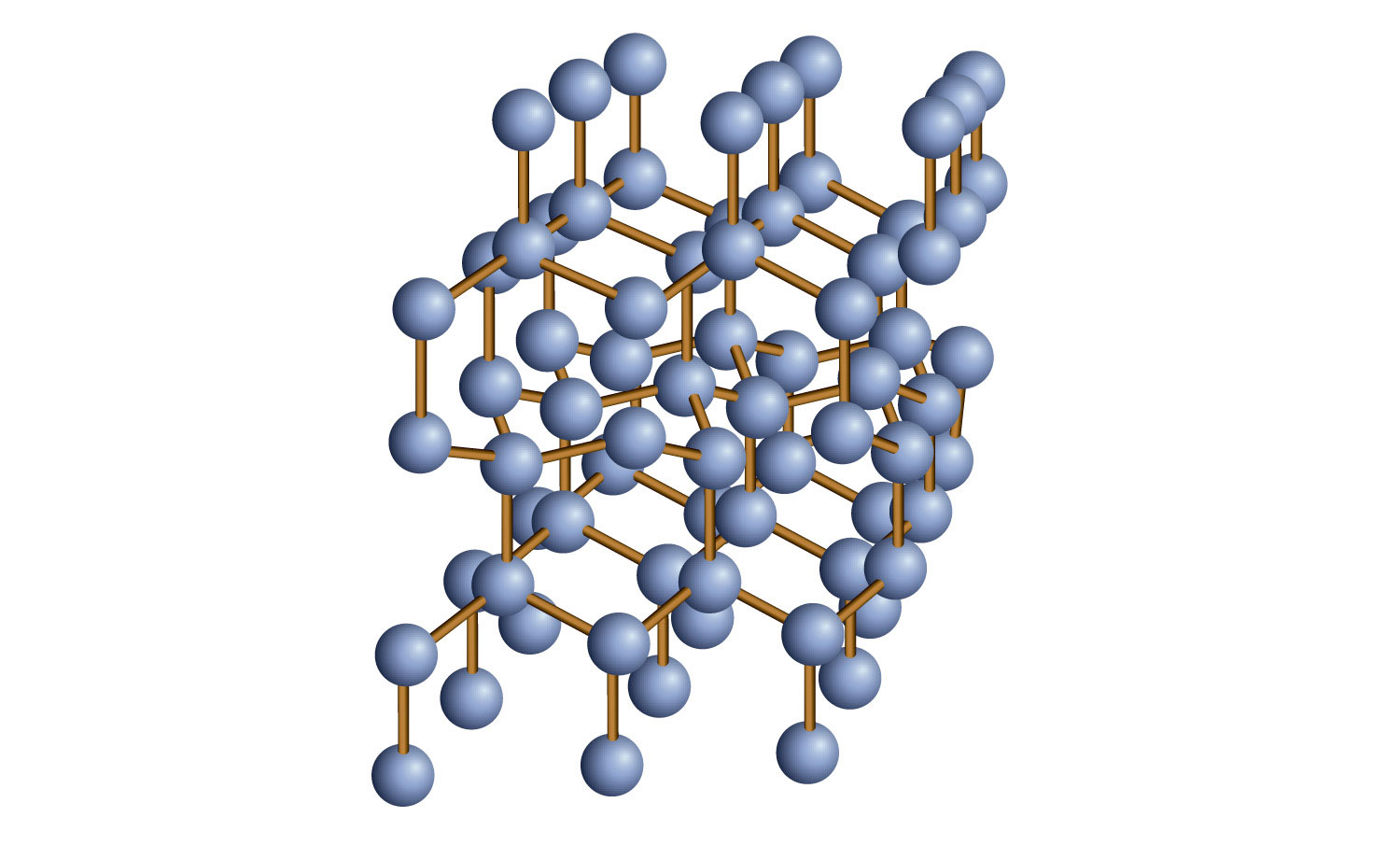
Covalent-network also called atomic solidsMade up of atoms connected by covalent bonds. Give an example of a network solid and describe the bonding in such a solid. Quartz is a network solid made of continuous SiO 2 subunits.
Add your answer and earn points. A netoccupational solid does not have actually discrete molecules. Examples of network solids include diamond with a continuous network of carbon atoms and silicon dioxide or quartz with a continuous three dimensional network of SiO 2 units.
So uh were asked about what a network solid is. A network solid is a solid in which all the atoms are covalently bonded to each other. Assume the density of the river water was 101gmL.
The sample was heated to dryness. An example of a molecular solid is sucrose. All groups and messages.
The drought most commonly known covalent network solids are unique in beauty diamond type and silicon dioxide SiO 2.

0 Response to "Describe a Network Solid and Give Two Examples"
Post a Comment